Humans, Sharks Share Immune-System Feature
DENVER —
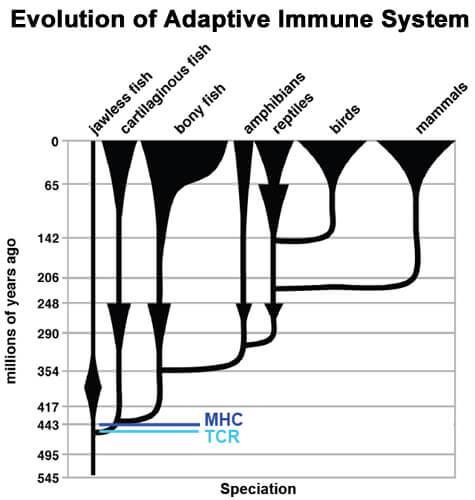
A central element of the immune system has remained constant through more than 400 million years of evolution, according to new research at National Jewish Health. In the September 29, 2011, online version of the journal Immunity, the researchers report that T-cell receptors from mice continue to function even when pieces of shark, frog and trout receptors are substituted in. The function of the chimeric receptors depends on a few crucial amino acids, found also in humans, that help the T-cell receptor bind to MHC molecules presenting antigens.
"These findings prove a hypothesis first proposed 40 years ago,” said senior author Laurent Gapin, PhD, associate professor of immunology in the Integrated Department of Immunology at National Jewish Health and the University of Colorado Denver. “Even though mammals, amphibians and cartilaginous fish last shared a common ancestor more than 400 million years ago, they continue to share an element of their T-cell receptors, indicating that the T cell-MHC interaction arose early in the evolution of the immune system, and is central to its function.”
The T cell serves as the sentinel, manager and enforcer of the adaptive immune response. It relies on its receptor, the T-cell receptor, to recognize foreign material and identify the target of the immune-system attack. When the receptor binds to small fragments of foreign organisms, called antigens, the T cell becomes activated, proliferates and initiates an attack against any molecule or organism containing that antigen.
T cells, however, cannot recognize free-floating antigens. They recognize antigens only when they are held by MHC molecules on the surfaces of other cells, much as a hotdog bun (MHC molecule) holds a hotdog (antigen). This interaction between the T cell and MHC molecules is crucial for immune defense and organ transplants. Compatibility of transplanted organs is determined by the similarity of different people’s MHC molecules. Nonetheless, this interaction has long mystified scientists and is poorly understood.
In 1971 future Nobel Laureate Niels K. Jerne proposed that evolution might have selected for genes that specifically recognize MHC molecules. Evidence discovered later suggested T cells’ affinity for MHC molecules might instead be the product of development that occurs as T cells mature in the thymus. The question remained unanswered for 40 years.
The T-cell receptor is constructed by piecing together several peptides among dozens that are available, plus a few random amino acid sequences. This combination is what allows the immune system to generate an almost infinite variety of receptors capable of recognizing almost any potential invader. The receptor has six loops that are the primary binding points for the antigen-MHC complex. One of those loops, known as CDR2, frequently binds the MHC molecule.
Searching for possible similarities in T-cell receptors of different animals, the researchers compared the amino acid sequences of one segment of the T-cell receptor containing the CDR2 loop. Although the segments contained less than 30 percent of the same amino acids, two specific amino acids were the same in human, mouse, frog, trout and shark T-cell receptors. Those appeared to be amino acids specifically involved in binding to the MHC molecule.
“The evolutionary inheritance of this pattern goes all the way from sharks to humans, which last shared a common ancestor 450 million years ago,” said co-author Philippa Marrack, PhD.
The researchers then inserted segments containing the CDR2 loop from frog, trout and shark T-cell receptors into mouse cells. These chimeric T-cell receptors recognized antigen bound to a mouse MHC molecule.
Since sections of frog, trout and shark T-cell receptors functioned perfectly well in mice T-cell receptors, the experiments suggested that the T-cell’s ability to see an antigen only when complexed with an MHC molecule first arose more than 400 million years ago, when all four animals shared a common ancestor.
Media Resources
We have many faculty members, from bench scientists to clinicians, who can speak on almost any aspect of respiratory, immune, cardiac and gastrointestinal disease as well as lung cancer and basic immunology.
Media Contacts
Our team is available to arrange interviews, discuss events and story ideas.
- Adam Dormuth
303.398.1002 office
970.222.5034 mobile
dormutha@njhealth.org - Jessica Berry
303.398.1082 office
303.807.9491 mobile
berryj@njhealth.org
